Fantastic Info About How To Write Valence Electron Configuration

For example, the electron configuration.
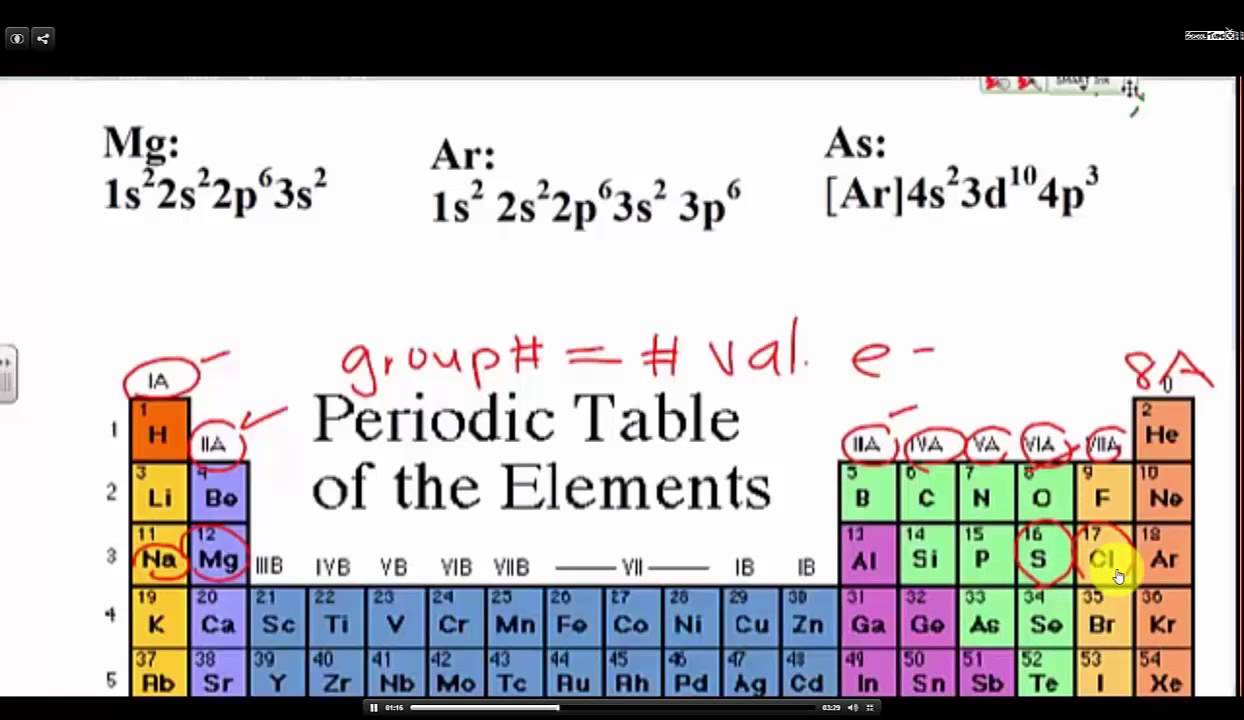
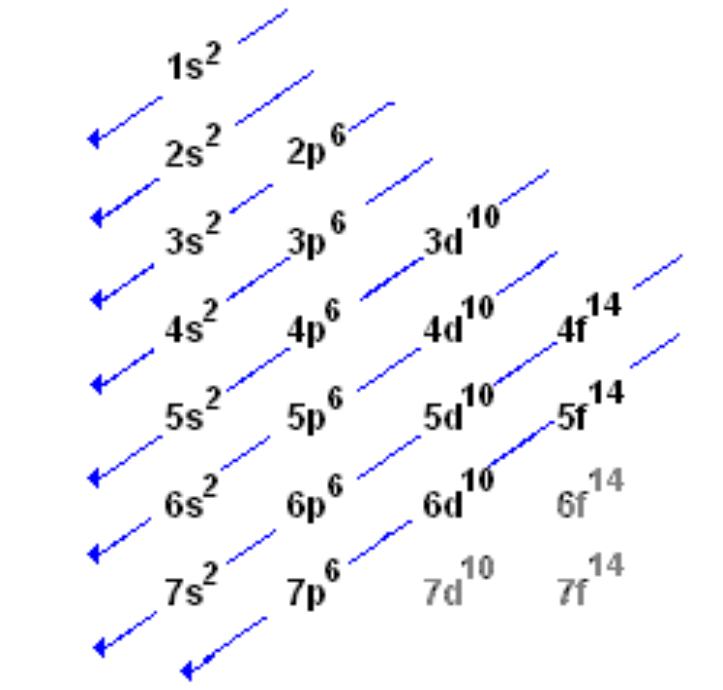
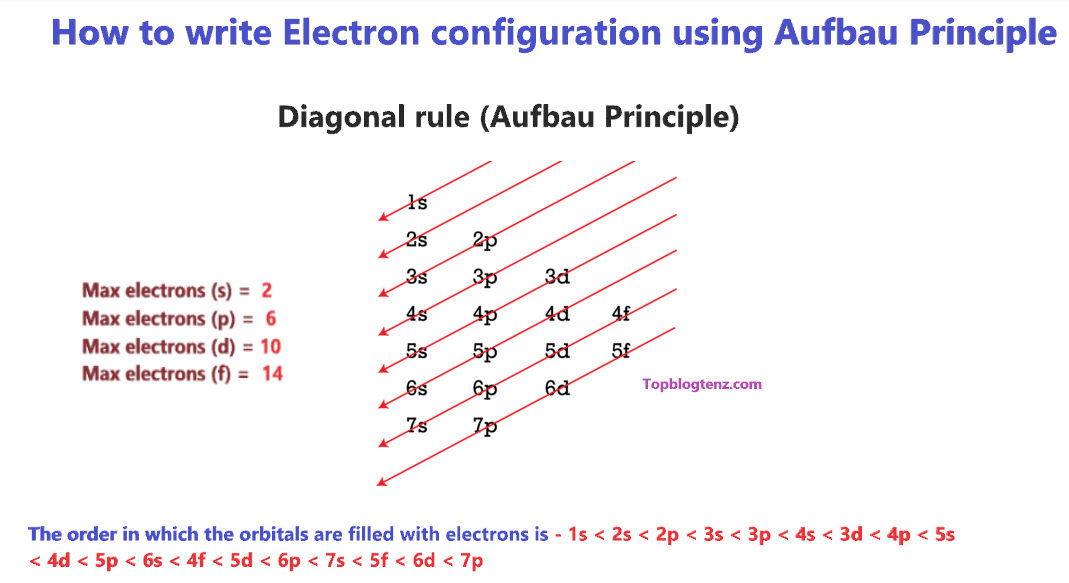
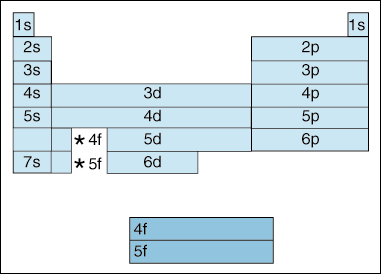
How to write valence electron configuration. So, the remaining four electrons will enter the 5d orbital. In writing the electron configuration for vanadium, the first 2 electrons will go into the 1s orbital, the next two electrons will go into the 2s orbital, and after that, the next 6 electrons will go into. The noble gas configuration for the scandium atom is written as [ar] 4s2 3d 1.
Palladium atom donates two electrons in the 4d orbital to convert a. The elements that form bonds by donating electrons are called cation. Therefore, the order of the number.
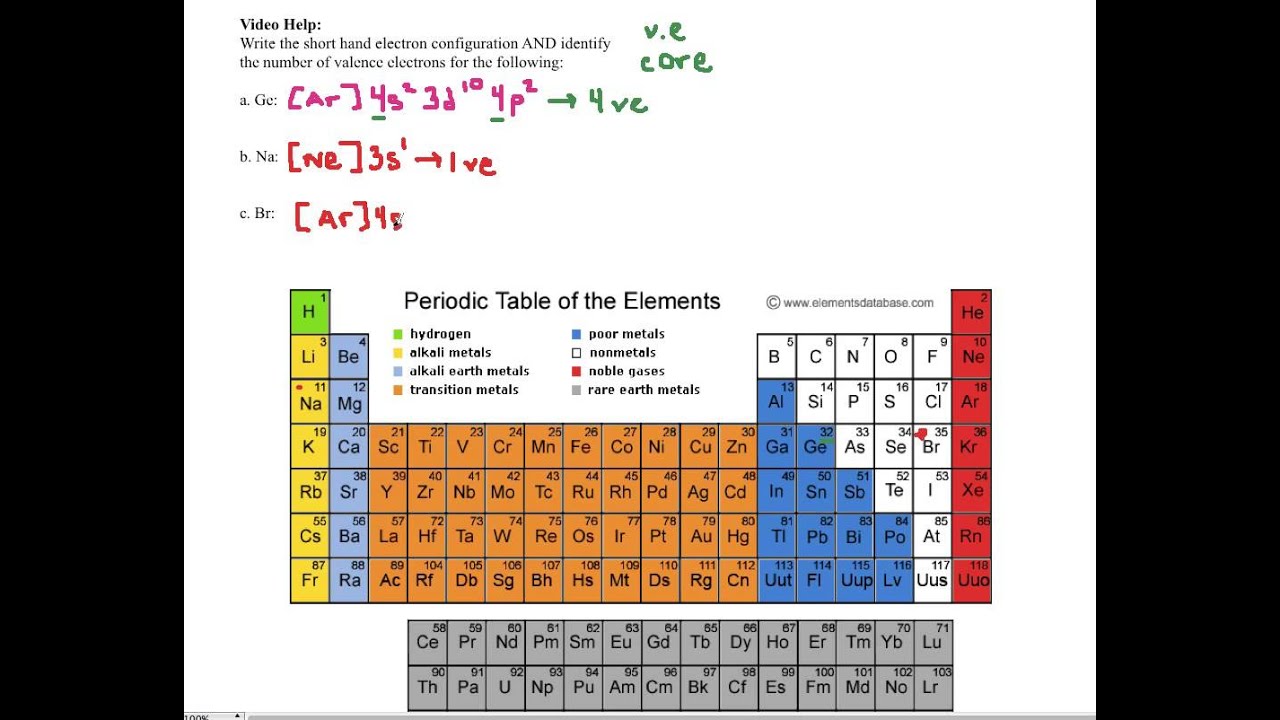
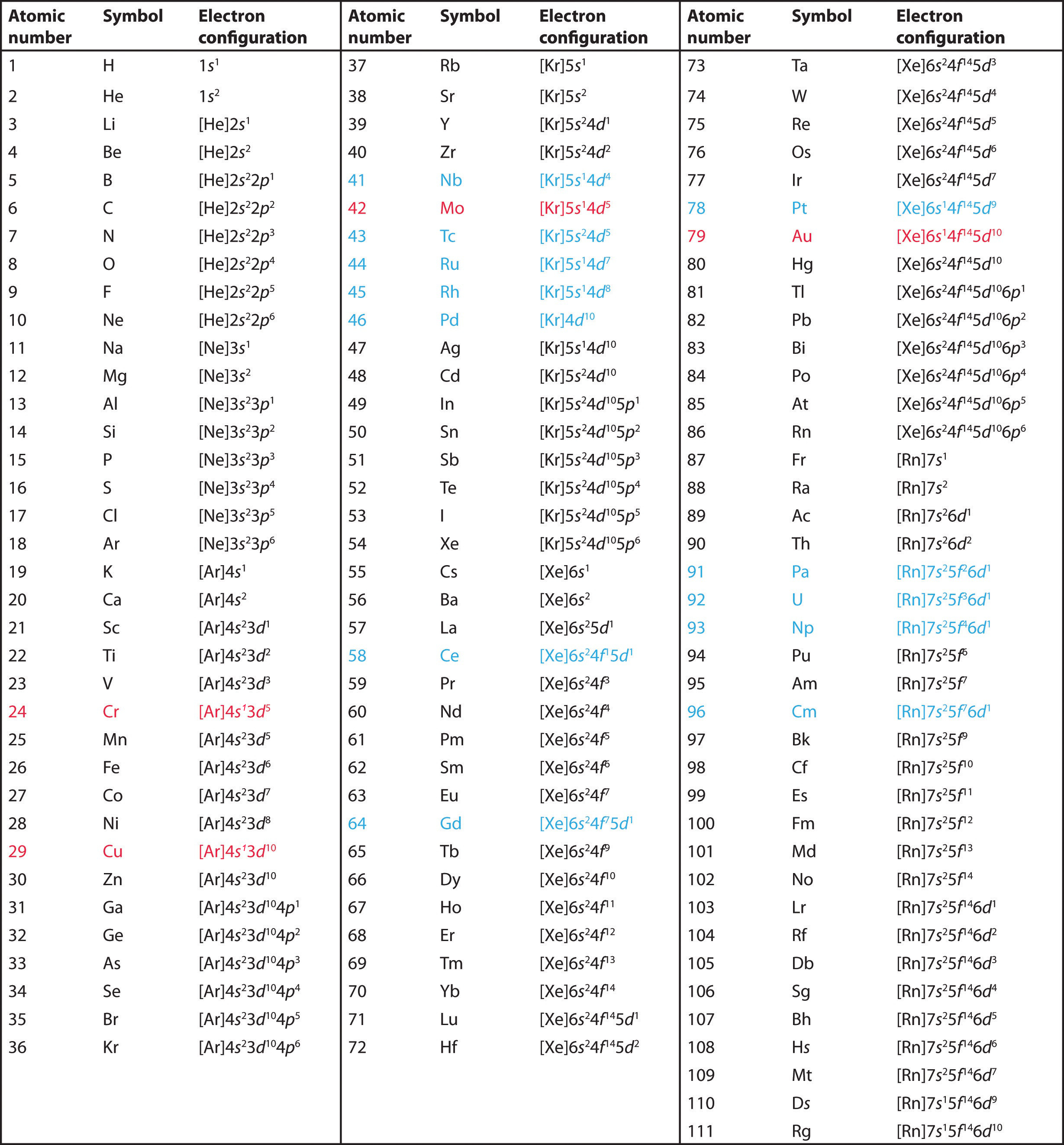
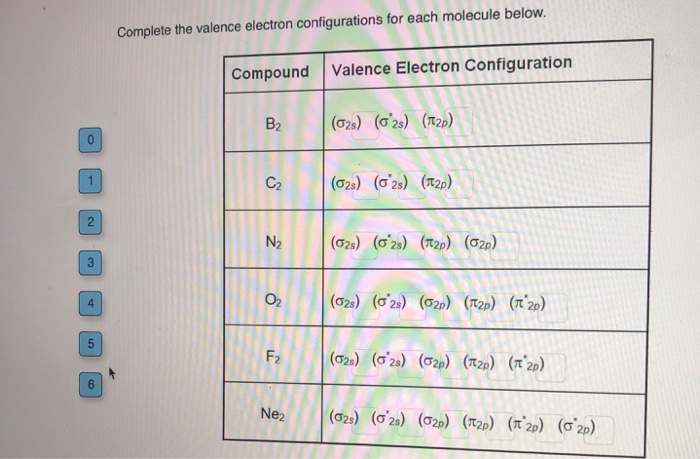
Therefore, the valence electrons of palladium are ten. Write out the electron configuration for the following elements: Write the valence electron configuration of each element by first indicating the filled inner shells using the symbol for the nearest preceding noble gas and then listing the principal quantum.
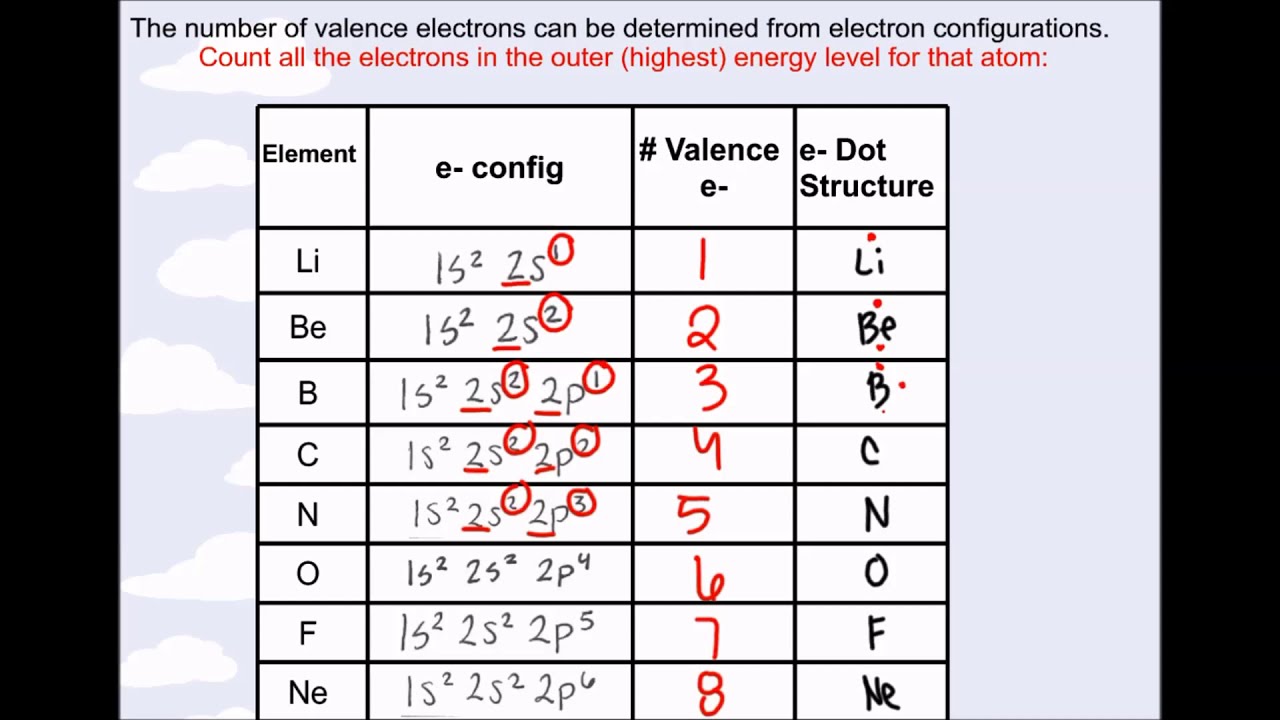
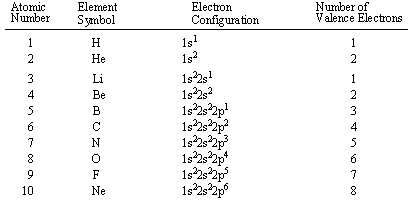
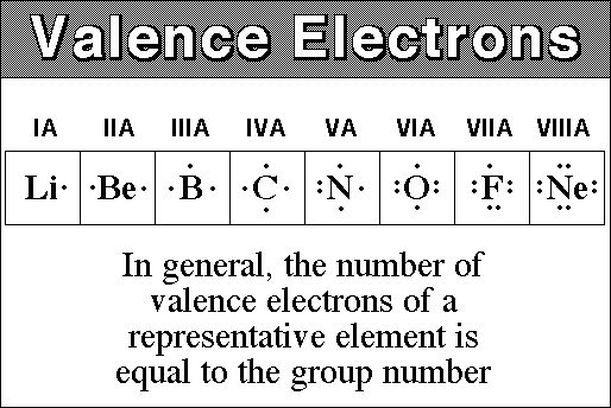
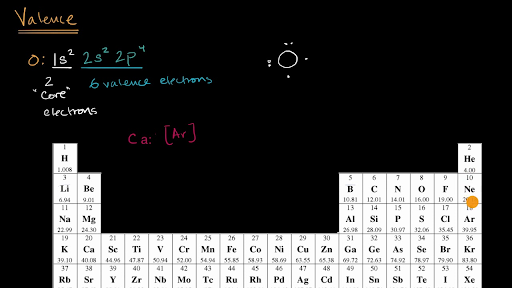
There is a shorthand way of writing electron configurations. Let’s find the valence electrons of the nitrogen atom through its electron configuration. Here, the electron configuration of vanadium ion (v 2+) is 1s 2 2s 2 2p 6 3s 2.
In the lewis symbol for an atom, the chemical symbol of the element (as found on the periodic table) is written, and the valence electrons are represented as dots surrounding it. In electronic configuration electrons are arranged in. Therefore, the electron configuration of bromine(br*) in an excited state will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p x 2 4p y 1 4p z 1 4d xy 1.
The vanadium atom donates two electrons from the last shell to form the vanadium ion (v 2+ ). This will be where its outermost or valence, electrons will be. The valency of the element is determined by.